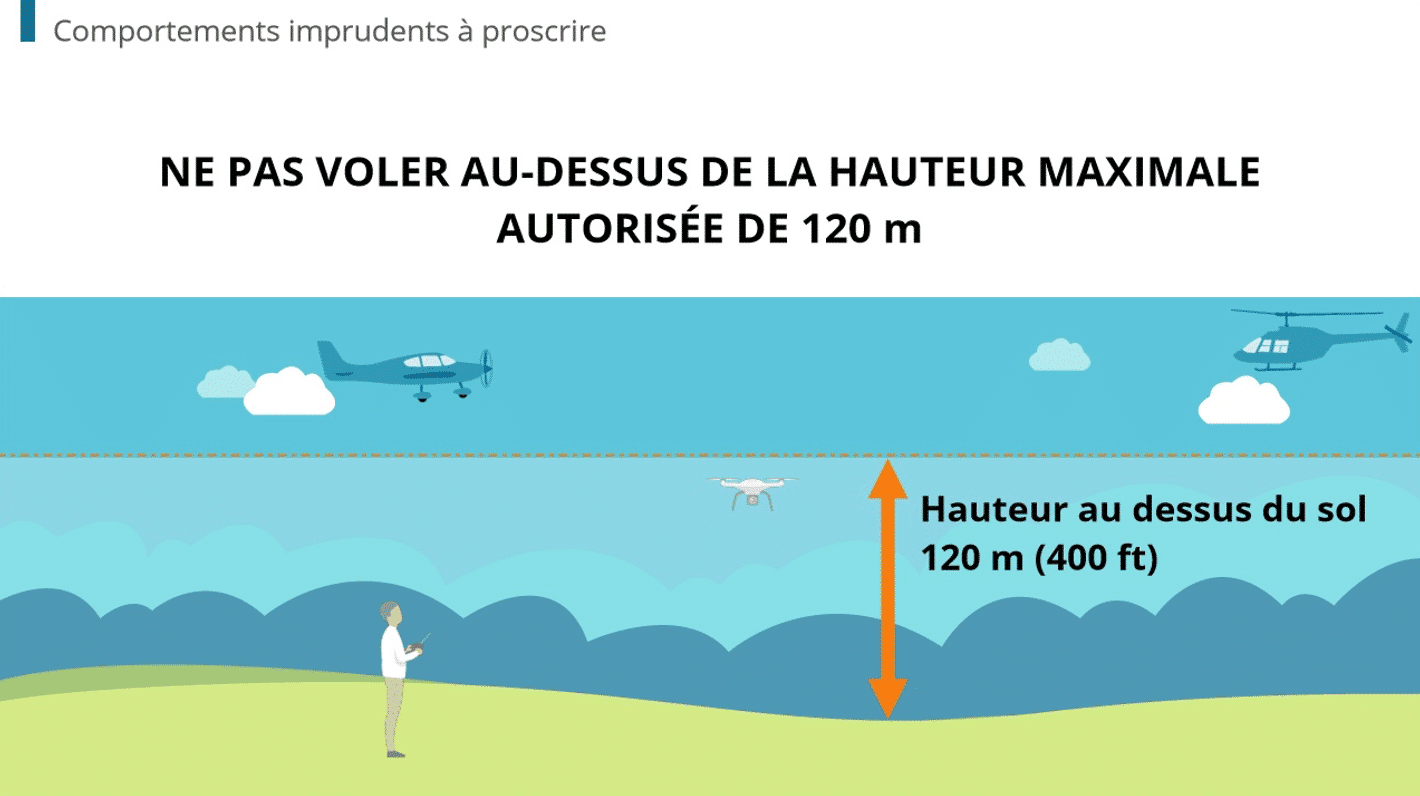
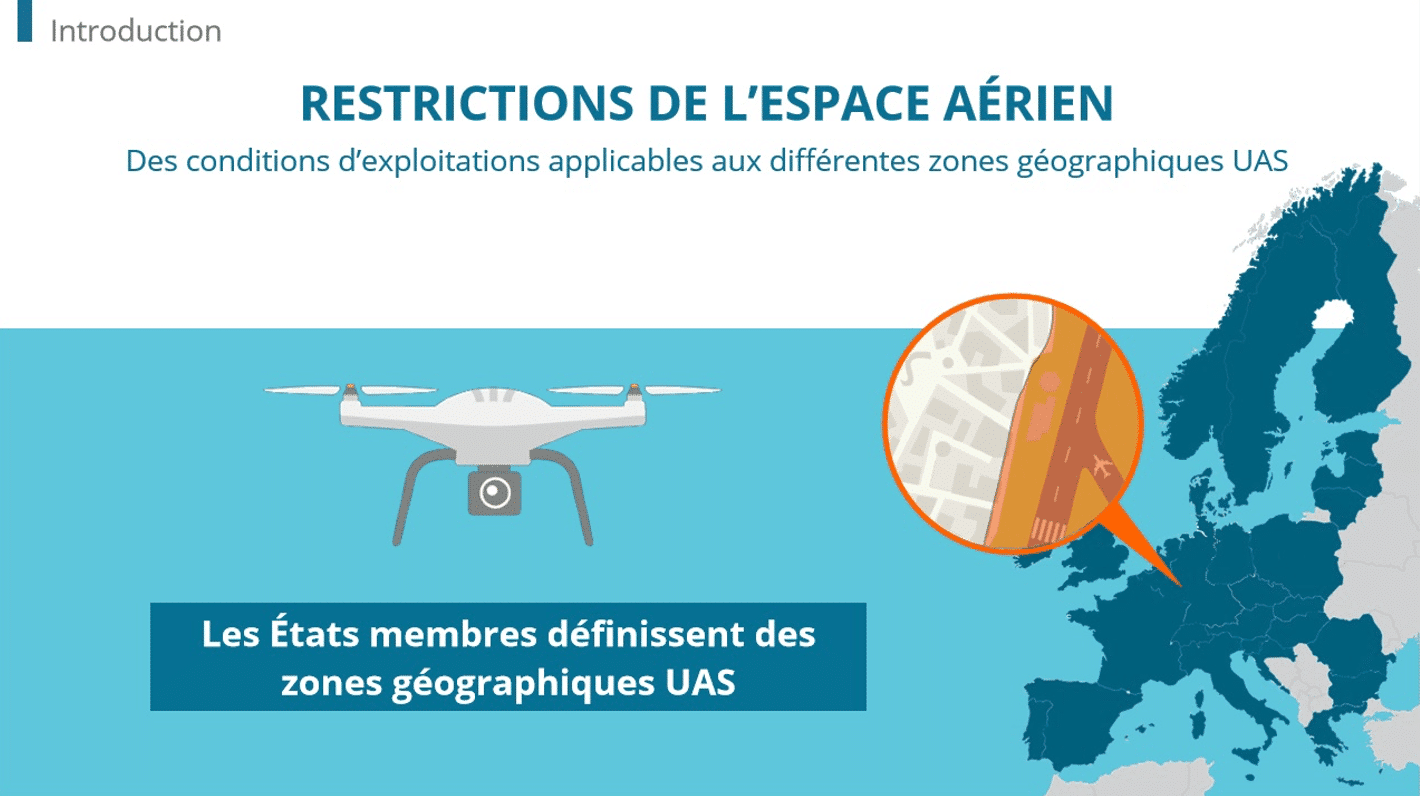
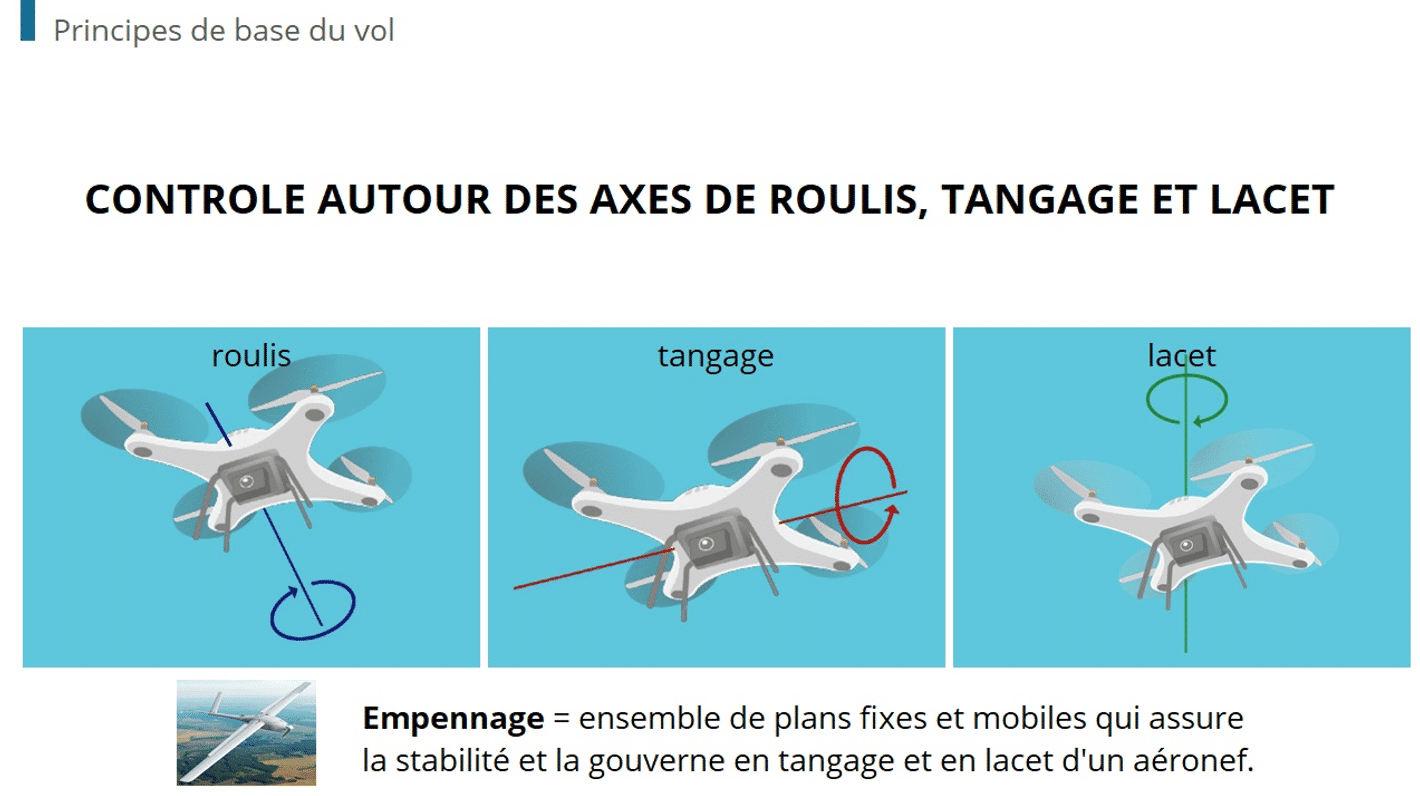
How An LMS Meets The Training Challenges In Medical Device Manufacturing

The medical device market size in the U.S. is set to grow to $314.96 billion by 2032, highlighting the industry’s rapid growth and need for a strict regulatory environment. Comprehensive and up-to-date training is vital to ensure compliance, product quality, innovation, and operation excellence. The requirement for a strict regulatory environment gives rise to the […]
What is a validated LMS ?

FDA 21 CFR 11.10(a) explains that a validated system is required to guarantee the accuracy, reliability, and consistency of electronic records and processes. For pharmaceutical companies, validation is a non-negotiable requirement. How are systems validated? Does training content need to be validated? This article unpacks the following subjects: Let’s dive in. What is a Validated […]
Training Management System (TMS) Vs. Learning Management System (LMS)

The pharmaceutical and biotechnological industries are accustomed to change. Regulatory updates, new trends, and emerging information are a constant in this industry. In 2024 alone, the CFR Title 21-Food and Drugs saw approximately 229 section updates, emphasizing the importance of keeping employees informed and trained. What software applications—platforms—are available to address your organization’s training requirements […]
Effective GMP Compliance: Ensuring Quality Control in Pharmaceutical Manufacturing

In the manufacturing sector, GMP, or Good Manufacturing Practices, is more than just a buzzword. It’s a set of protocols and operations that should be company doctrine for all businesses involved in the pharmaceutical manufacturing, testing, and release of end-user products. Find out how to implement and enforce GMP compliance and promote quality control. The […]
Strengthening Pharma Compliance: CAPA Training for Quality Standards

Corrective action and preventive action, or CAPA, is an organizational method for identifying, investigating, and resolving issues that may manifest during a product development, testing, or release phase. Pharma manufacturing and medical device industries are mandated to have a CAPA plan in place under the Food and Drug Administration (FDA) and International Organization for Standardization […]