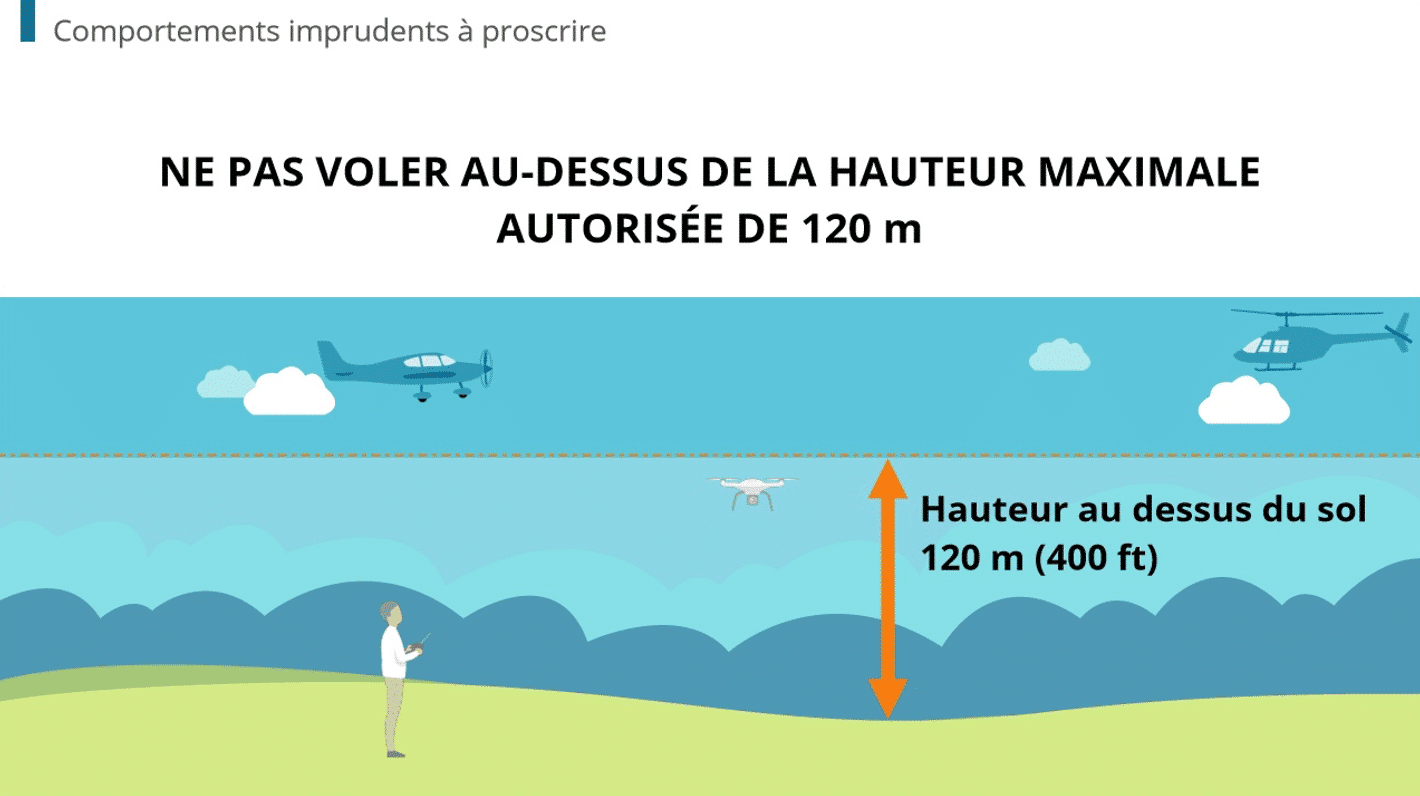
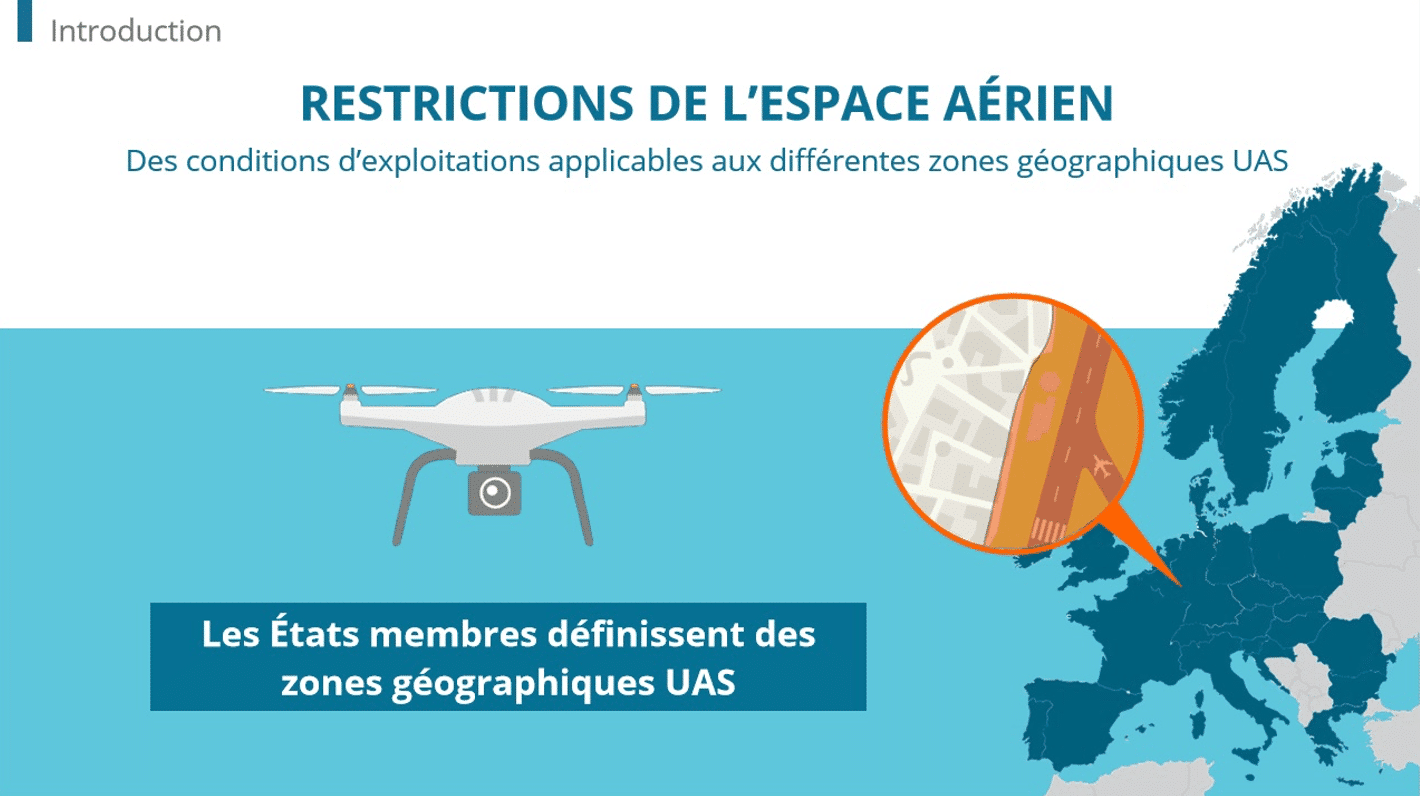
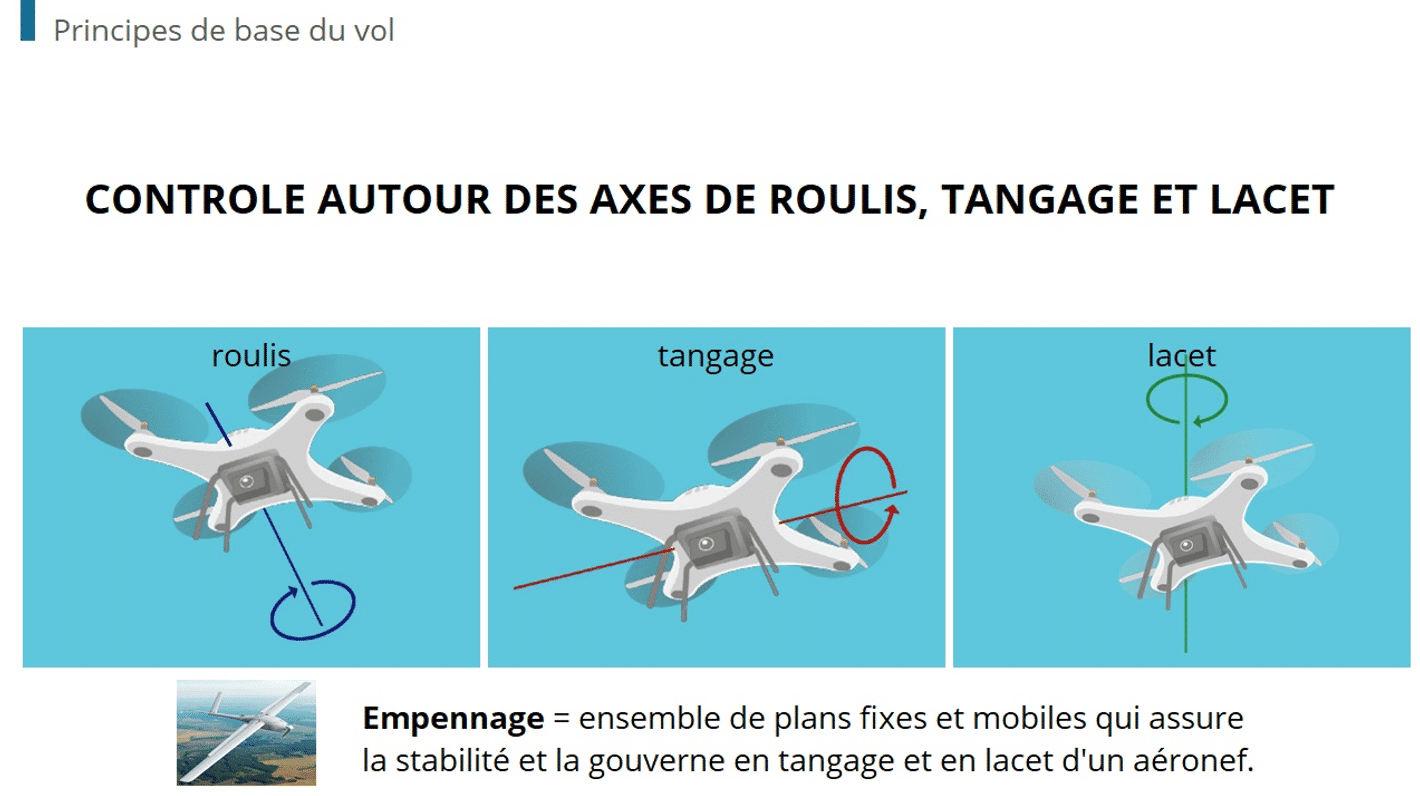
4 Strategies For Empowering Manufacturing Teams With Continuous Skill Development

Upskilling employees with continuous development is vital for building a business that prioritizes operational performance and competitiveness in the pharmaceutical industry. Keeping up with new technologies and industry regulations requires manufacturing companies to invest in continuous workforce education. The global healthcare Learning Management System (LMS) is projected to surpass $3.5 billion by 2030—highlighting the growing […]
Upskilling in Manufacturing: How Digital Learning Transforms Workforce Efficiency and Compliance

With a market value exceeding $1 billion in 2024, pharmaceutical manufacturing continues to grow. However, regulatory compliance—particularly with 21 CFR Part 11—remains a persistent challenge. One way to address this is through structured employee training and upskilling. Our experts discuss how digital learning improves workforce efficiency and ensures compliance. We will focus on how Dokeos […]
What is a validated LMS ?

FDA 21 CFR 11.10(a) explains that a validated system is required to guarantee the accuracy, reliability, and consistency of electronic records and processes. For pharmaceutical companies, validation is a non-negotiable requirement. How are systems validated? Does training content need to be validated? This article unpacks the following subjects: Let’s dive in. What is a Validated […]
How to build a Training program that meets FDA Standards? A Comprehensive Guide for Quality Managers

The global pharmaceutical industry is estimated to reach $1.6 trillion in 2025. Ensuring regulatory compliance for industry operations is essential for safeguarding patient health and maintaining operational efficiency and reputation. The importance of regulatory complaint training cannot be understated in pharmaceutical operations. In this article, our quality experts detail how quality managers can build training programs […]
Selecting a validated LMS for manufacturing: ensuring SOP compliance and workforce training precision

The global pharmaceutical industry generated $576.64 billion in 2024 and is projected to grow to $929.9 billion by 2030. While these metrics highlight the sector’s rapid expansion, patient safety remains the top priority. Calling attention to the need for manufacturers to prioritize risk management, quality assurance, and safety protocols. How can manufacturers take advantage of […]
The Importance of LMS Validation in Enhancing GMP Training in the Pharmaceutical Industry

What happens when Good Manufacturing Practices are ignored? Between 2013 and 2022, inspections revealed over 1,400 deficiencies, with hundreds classified as major or critical. These findings emphasize the urgent need for thorough training and compliance in pharmaceutical manufacturing. To meet regulatory requirements, companies must provide employees with precise, up-to-date training while keeping detailed records for […]
From Compliance to Efficiency: How SOPs Drive Pharmaceutical Manufacturing Quality and Consistency

It is said that human error may be responsible for more than 80% of process deviations in pharmaceutical manufacturing. Consider the challenge of producing pharmaceutical products that are high-quality and consistent in the absence of standardized procedures. The result? A recipe for avoidable expenses, miscommunication, human error, and non-compliance. Standard Operating Procedures (SOPs) are critical […]
Medical Affairs: Building Secure, Compliant and Efficient Prescriber Training with Dokeos LMS

Prescribing is a complex task that requires HCPs to be equipped with the necessary skills and knowledge to prescribe medical products effectively and without bias. Prescribing decisions directly impacts patient outcomes, highlighting the imperative for continuous education and ongoing support for healthcare professionals (HCPs). Studies describe how HCPs are more likely to prescribe a particular […]
Training Management System (TMS) Vs. Learning Management System (LMS)

The pharmaceutical and biotechnological industries are accustomed to change. Regulatory updates, new trends, and emerging information are a constant in this industry. In 2024 alone, the CFR Title 21-Food and Drugs saw approximately 229 section updates, emphasizing the importance of keeping employees informed and trained. What software applications—platforms—are available to address your organization’s training requirements […]
5 Best Practices For Managing Training Records In Medical Device Manufacturing

So far, in 2024, there have been 100 medical device and radiation-emitting product recalls, highlighting the ongoing challenge medical device manufacturers face in maintaining device quality, safety, and efficacy post-approval. How do you prioritize quality throughout the supply chain? Through training records. Medical device companies must establish protocols to identify employee training requirements and to […]