A Modern GxP Validated LMS for Life Sciences Organizations
Combine learning, document, and quality management in one platform to deliver effective training while maintaining full regulatory compliance.
Trusted by Pharmaceutical and Biotech Organizations








Transform Pharmaceutical Training with Dokeos LMS

Complete Regulatory Compliance
Our validated system ensures data integrity with comprehensive audit trails and electronic signatures, meeting the highest regulatory standards including FDA 21 CFR Part 11 requirements.
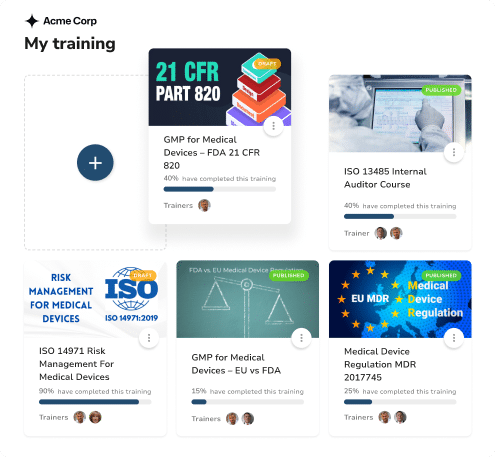
Modern Interface
Experience a responsive, intuitive interface that works seamlessly across all devices, making learning engaging and accessible whenever and wherever your teams need it.
End-to-End Training Management
Support every phase of your life sciences operations with unified training management, from clinical trials and manufacturing to medical device operations and sales force education.
Comprehensive Life Sciences Coverage
Dokeos LMS supports every phase of pharmaceutical and biotech operations:

Clinical Research
Protocol training management, Investigator qualification tracking, Study documentation compliance, Multi-site training coordination.
Drug Manufacturing
GMP compliance training, SOP management, Quality control procedures, CAPA training tracking.
Medical Device Operations
ISO 13485 compliance, Device training management, Technical documentation, Service team education.

Sales & Medical Affairs
Product knowledge training, HCP education programs, Sales force certification
Discover 80+ Certified Courses for Compliant Training
Access our complete library of GxP training content tailored for quality assurance teams. Get ready for any regulatory inspections auditing your internal processes.
Meet our compliance experts
Dokeos’ experts deliver tailored strategies to ensure regulatory alignment and audit readiness.
Dr. Patrick Jonvel
40 years in pharma with ALCON, ELI LILLY, MERCK & IPSEN. Expert in quality, compliance, and training, with 15+ FDA inspections and no major findings. Created 100+ courses and delivered 40K+ hours of GLP/GMP training.
Dr. Thomas De Praetere
Founder of Dokeos, has 20+ years of experience in learning technologies and regulated industries, specializing in pharma and medical devices. As a consultant Quality Manager at Dokeos, he ensures GxP and FDA compliance for training systems in the life sciences sector.
Christine Amory
Christine Amory, Chief Customer Officer at Dokeos, has 15+ years of experience in e-learning and regulatory compliance for pharma and healthcare. She specializes in developing tailored training programs and ensuring adherence to standards like HIPAA and GDPR.
Stephan Atsou
Stephan Atsou, CEO of Dokeos, brings 25 years of expertise in digital learning and corporate training. Formerly leading CrossKnowledge Benelux, he specializes in aligning learning strategies with business goals and is co-author of a guide on e-learning for companies.
Still have questions?
With decades of experience in regulated industries, we speak your language. Let’s discuss your specific needs.
Trusted by Pharmaceutical and Biotech Organizations

















































