Transform Your Medical Education Strategy with Dokeos LMS
Elevate your medical affairs programs with a LMS designed for healthcare professionals education. Dokeos LMS combines robust traceability features with engaging educational capabilities to help you deliver impactful medical education.
Trusted by leading pharmaceutical and medical device companies








Strategic Medical Education Solutions
Medical Science Liaisons are the crucial bridge between scientific advancement and healthcare delivery. Empower your MSL teams with training tools designed specifically for scientific exchange with Key Opinion Leaders, ensuring your therapeutic expertise reaches healthcare professionals effectively. Our platform helps you:

Deliver impactful scientific education

Engage healthcare professionals effectively

Track educational program success

Measure knowledge retention
Why Do Medical Affairs Teams Choose Dokeos?
Drive end-to-end medical education excellence with a platform that supports your complete training journey. Whether you’re developing internal talent or educating healthcare professionals about your products, Dokeos delivers the tools you need for successful vertical integration of your medical affairs strategy.

Modern Learning Experience
- Engaging, multimedia-rich educational content
- Mobile-responsive design for on-the-go access
- Interactive assessment capabilities
- Seamless virtual meeting integration
Comprehensive Analytics
- Detailed engagement metrics
- HCP participation tracking
- Educational impact measurement
- Program effectiveness analysis

Traceability for Non-corruption
- Transparent documentation of all educational activities
- Complete reporting capabilities
- Ethical guidelines adherence tracking
Advanced Features designed for Medical Affairs Success
Equip your medical affairs team with powerful tools that drive measurable outcomes across your entire educational ecosystem – from internal knowledge development to impactful physician engagement. Our key features include:
- HCP Engagement Tracking
- Scientific Content Management
- Certification Management
- Educational Analytics
- Virtual Event Management
- Multi-Channel Support
- Complete visibility of educational activities
- Detailed participation metrics
- Engagement level analysis
- Automated follow-up management
- Version control for medical content
- Approval workflow automation
- Real-time content updates
- Multi-language support
- Digital certification issuance
- Expiration tracking
- Automated renewal notifications
- Verification portal for stakeholders
- Comprehensive learning metrics
- Content effectiveness analysis
- Knowledge retention tracking
- ROI measurement tools
- Seamless webinar integration
- Attendance tracking
- Engagement monitoring
- Interactive session tools
- Consistent cross-platform delivery
- Mobile-first design
- Offline access capabilities
- Synchronized learning progress
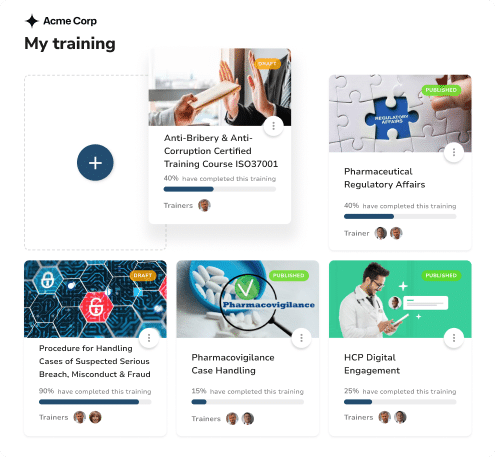
Discover 80+ Certified Courses for Medical Affairs
Access our extensive library of medical education templates and frameworks. Create consistent educational programs efficiently.
Frequently Asked Questions
Our platform offers multiple engagement channels, interactive content, and personalized learning paths to maximize HCP engagement and knowledge retention.
Yes, comprehensive analytics track engagement, knowledge retention, and program effectiveness, helping you demonstrate the value of your medical education initiatives.
The platform includes multi-language support, regional content adaptation capabilities, and global program management tools to ensure consistent education delivery worldwide.

Still have questions?
Fill out this form to contact us. An expert in Medical Affairs will get back to you shortly.
Trusted by leading pharmaceutical and medical device companies


















































