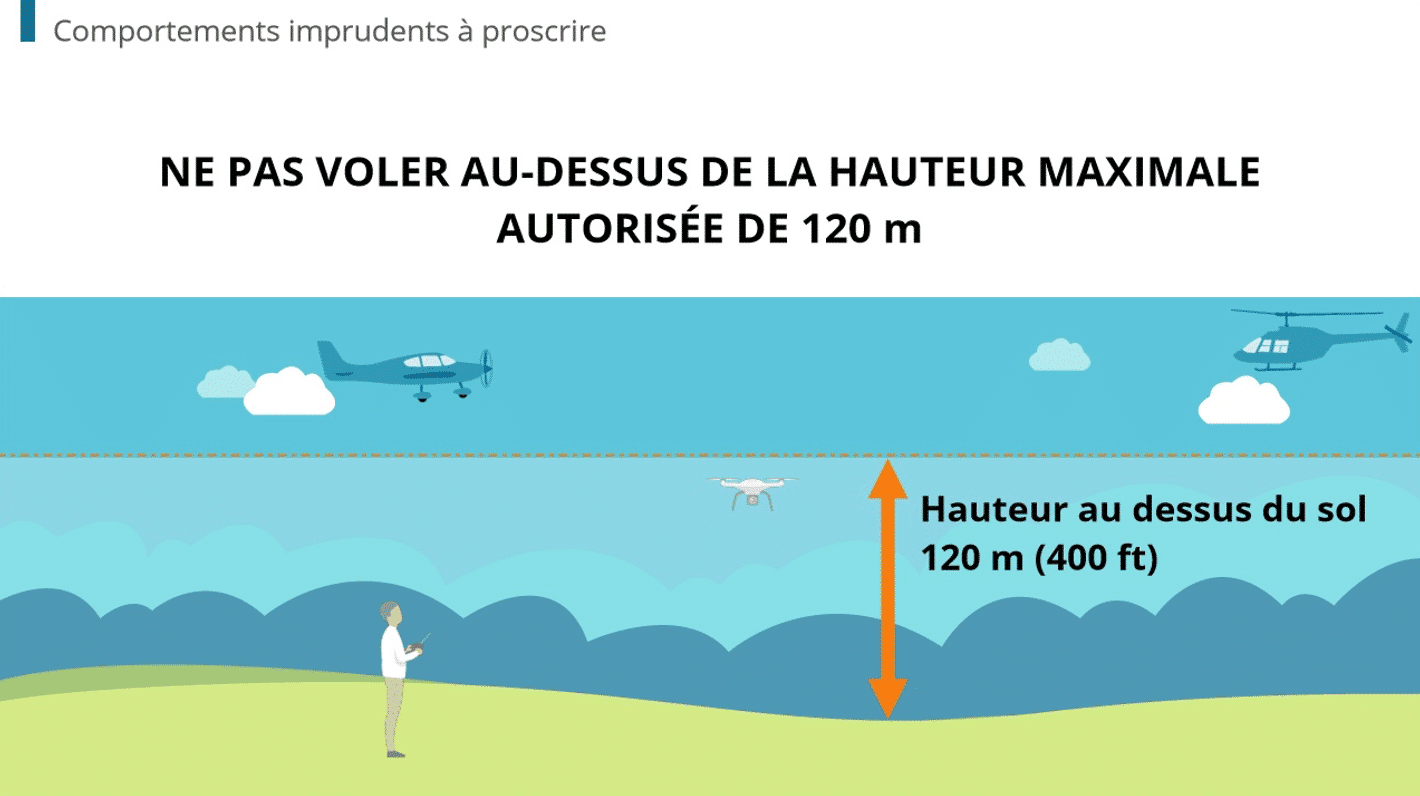
All medical e-learning solutions must comply with the FDA standard 21 CFR PART 11. This is the case with Dokeos LMS.
One of the strengths of Dokeos LMS is its compliance with the FDA (Food and Drug Administration) standard 21 CFR PART 11. Compliance with this standard is in fact necessary for all companies within the food, clinical or pharmaceutical industries, including within the framework of their e-learning training. There are three aspects of this regulation that relate particularly to e-learning: traceability, data authentication and users.
You can also learn thanks to our partner GxP Training : Discover FDA 21 CFR PART 11 Online Course and Certifcation.
The 21 CFR Part 11 regulation
The American standard 21 CFR Part 11 requires ensuring and documenting the traceability of actions carried out within an electronic information system. It can therefore provide detailed and authenticated information on the history of changes made to it. This obligation includes e-learning systems for training medical, pharmaceutical and food industry staff. As the American market is essential for all drugs manufacturers, compliance with the FDA standard 21 CFR Part 11 has been quickly established outside of American borders.
Dokeos and traceability required by the 21 CFR Part 11 standard
An e-learning solution must be able to provide a precise set of personalised data for each learner and training session. This is the case with the Dokeos LMS reporting, which allows each learner to obtain detailed information:
- The results of a quiz.
- The results of an exam.
- The number of questions answered in a quiz or exam.
- The time spent on a quiz or exam.
- The number of attempts at a quiz or exam.
- The exam or quiz attempt dates
- SCORM module progress.
- Time spent on the SCORM modules.
- The quiz scores inserted into the SCORM modules.
- The login and logout dates in LMS.
- The session duration in LMS.
- The quiz modification dates.
- The survey content.
- The survey statistics.
- The survey date.
- The user profile, name of the user’s business unit or department.
- The name of the individual in charge of training.
- The average result of the training group, its progress and the time spent.
- The presence or not of the user in individual interviews.
- The date of issuance of certificates.
Authentication and securing of critical data
Another important aspect of the regulation for e-learning is the possibility of making “hard copies”. In order to ensure the data authenticity of an information system, electronic registration is not sufficient from a legal point of view. This is why the results of all the Dokeos reporting data can be printed and dated before being authenticated.
Authenticating users
Finally, Dokeos allows various procedures to be used for improving user authentication: mandatory complex passwords, regular password changes, account blocking after several unsuccessful connection attempts with warning sent by the administrator.
Used by many companies working in the medical and clinical research industry, Dokeos ensures perfect compliance with the FDA standard 21 CFR Part 11. Is the Dokeos solution of interest to you? Discover Dokeos free of charge for 60 days!