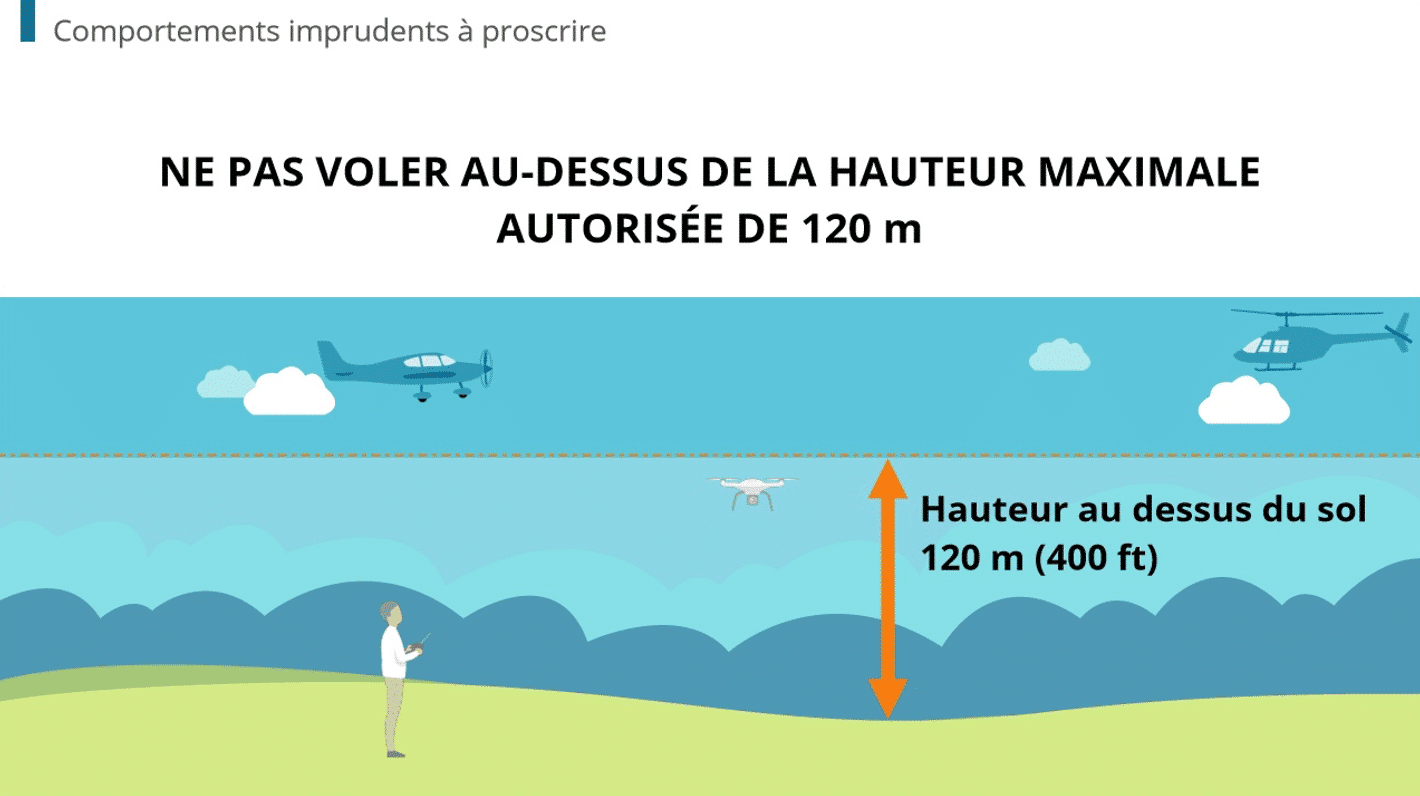
The pharmaceutical industry has stringent regulatory requirements, one of which is audit trail management. An audit trail provides a secure and detailed chronological record of user activities. This is often in relation to the lifecycle of a critical product, as is often the case in pharmaceutical manufacturing.
The primary purpose of audit trail management in pharmaceuticals is to ensure data integrity and accountability. This article will cover audit trail management and explain why learning management systems (LMSs) are important in the pharmaceutical industry.
Difficulties in pharma audit trail management
The U.S. Food & Drug Administration (FDA) requires pharmaceutical companies to plan and execute a monitoring system to ensure adequate process performance and product quality. As a result, it states that a formal process to review the pharmaceutical quality system needs to be in place.
This process involves risk assessment and audit trail management. The goal is to provide the best standards established with regard to critical components in the manufacture and lifecycle of pharmaceutical products.
The FDA’s 21 CFR Part 11 is a rigorous regulation that outlines “procedures to follow regarding document security and electronic signatures.”
For example, it says that electronic signatures must be linked to their corresponding electronic records in order to prevent the false or inappropriate transfer or copying of the signatures.
Previously in the pharma industry, you could either use a manual, paper-based system or an electronic one.
But paper-based records are more prone to error because of manual entry and can be easily discarded. This makes audits arduous because of the lack of data availability.
To that end, the FDA has outlawed the use of paper-based audit trails.
Features of a pharmaceutical LMS that simplify audit trail management
A major benefit of an LMS is the administration and automated delivery of educational courses. However, apart from ensuring adequate employee training and compliance, an LMS provides quality and risk management processes such as audit trail management.
This is a huge boost since audit trail management has become a regulatory requirement in the pharmaceutical industry.
Therefore, pharma companies must now incorporate a strategic view of LMS functions as an invaluable tool for compliance, training, quality assurance, and risk management.
Moreover, beyond benefits and financial incentives, employees are increasingly looking for opportunities for personal growth. Providing learning management systems engages them and makes them feel that they matter.
A pharmaceutical LMS matters because it demonstrates that pharmaceutical companies are keen on maintaining staff training. It also provides rigid audit trails that prevent situations that may lead to compliance failures—for instance, erroneously allowing backdated signatures on a document.
Continuous tracking
The use of an LMS in the pharmaceutical industry enables companies and employees to identify necessary job tasks and operations. This visibility not only demonstrates compliance training to auditors but also allows the regular tracking of all components that could be audited.
Custom report delivery
A pharma LMS provides training analysis reports to document work practices necessary for the audit trail. They often provide employee training on relevant good manufacturing practices (GMP), like delivery reports in the correct format.
In addition to maintaining standard reports, an LMS also has the flexibility and nimbleness to allow customizations so pharma regulator reporting requirements can be met.
Secure system for sensitive data
In this age of high-profile cybersecurity data breaches, the security of pharma data and proprietary manufacturing information should be paramount in the mind of executives.
Pharmaceutical LMS systems do a lot of things to maintain the integrity of the system’s data. For instance, stringent audit trail management ensures that it only takes valid inputs.
Data storage capabilities
Closely related to providing a secure data system is having adequate data storage capabilities. The advent of cloud computing has provided relatively cheap yet sophisticated storage systems.
These systems are robust and come with backup, fault tolerance, and redundancy to make stored data stored.
Most pharmaceutical LMSs have taken advantage of these cutting-edge technologies to boost their data storage capabilities.
Provision of robust, centralized data
Fortunately, the use of an LMS in the pharmaceutical industry creates a single repository of processes, documents, and training.
A pharmaceutical LMS helps greatly because it provides a centralized data repository. Because data is centralized and not dispersed, it offers better visibility. Therefore, it’s easier to manage, secure, and defend the data against unwanted intrusion.
Simplify your audit trail management with a pharmaceutical LMS
The pharma industry is saddled with the burden of strict and rigid regulation. However, audit trail requirements can be made easier with some of the features provided by a pharmaceutical LMS.
At Dokeos, we’re proud to be pioneers in social learning, having introduced the first collaborative learning tool.
Contact us today to discuss the implementation of a risk-based audit trail review process that meets regulatory requirements.